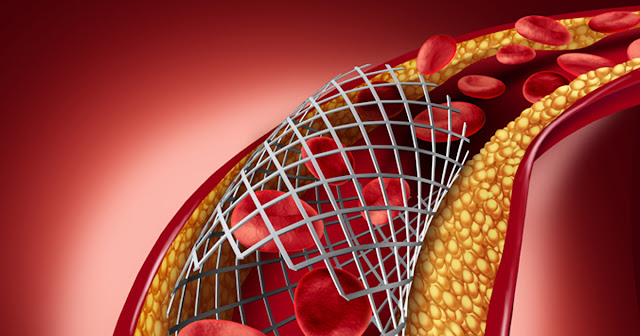
Coronary stents are a tiny metal mesh coil, usually inserted into the wall of a narrowed blood vessel in a procedure called percutaneous coronary intervention. The stent keeps the artery open after angioplasty, to reduce re-narrowing, and is used in around 95 per cent of PCI procedures. It has saved many patients from having to undergo coronary artery bypass surgery, which is more complicated and painful.
India Coronary Stents are used in a nonsurgical procedure called coronary angioplasty to treat heart disease and lower the risk for future heart attacks. Coronary arteries carry oxygen-rich blood to the heart, if they become blocked by a buildup of fatty plaque, it leads to a coronary artery disease. Over time, the heart muscle can’t get the blood and nutrients it needs to function normally, and this may lead to chest pain or a heart attack.
India Coronary Stents Market is estimated to be valued at US$ 1,303.5 million in 2023 and is expected to exhibit a CAGR of 13.2 % during the forecast period (2023-2030).
During a procedure, a long hollow tube (catheter) is inserted into the artery in the groin, wrist or arm and guided through X-rays to the narrowed area of the artery. A thin wire is then fed over the catheter and into the narrowed artery, and then a balloon with a squashed-down stent attached is inflated over the narrowed area of the artery. When the stent is in place, the cardiologist removes the catheter, balloon, and wire.
The stent remains in the artery to help prop it open and decrease its chance of narrowing again. Most stents are coated with a polymer that releases medication to help limit the growth of scar tissue that could block the artery again. Bare-metal stents are also available for patients who are allergic to the polymer or drugs in drug-eluting stents.
One of the key factors expected to hamper the growth of the India Hemostat Market is lack of awareness of bleeding disorders.
The stent is made of medical-grade stainless steel or platinum-chromium metal. It has a coating of a drug to stop scar tissue from growing inside the stent, which could otherwise lead to re-narrowing of the artery. These stents are known as ‘drug-eluting stents’ and were once less widely available in the NHS.
When India Coronary Stents were first introduced, research showed that they prevented heart attacks and deaths in some patients with stable coronary disease, and they also helped many people to have fewer symptoms of angina.
However, several randomized trials have since shown that, for most patients with stable CAD, stents do not significantly reduce the risk of a heart attack or death and might not even reduce the pain of angina.
An increase in government funding for development of neurology devices is expected to drive the growth of the global Neurology Devices Market over the forecast period.
Medtronic plc, gained approval for their Onyx Frontier drug-eluting stent, from the US FDA, in May 2022. This consists delivery system that enhances the acute performance and deliverability of the stent.



Comments
Post a Comment